The Role of Neuroinflammation in Neurodegenerative Diseases

Neuroinflammation is key in many neurodegenerative diseases like ALS, Huntington’s, and Alzheimer’s. It’s a complex area of study, focusing on how the immune system, glial cells, and neurons interact. This interaction is central to research in neurodegeneration.
Studies show that neuroinflammation is closely tied to the causes of these diseases. Researchers have found important processes like oxidative stress and protein aggregation. These processes harm neurons and lead to the symptoms of these diseases.
Grasping the details of neuroinflammation is vital for finding new treatments. By studying how the immune and nervous systems work together, scientists are finding new ways to help patients. This could lead to better treatments and outcomes for those suffering from these diseases.
Understanding the Basics of Neuroinflammation
Neuroinflammation is a complex process in the central nervous system (CNS). It involves many cellular components and inflammatory mediators. At the center of this process are microglia and astrocytes.
Cellular Components of Neuroinflammation
Microglia are the brain’s immune cells. They play a key role in neuroinflammation. When they are activated, they release cytokines and chemokines. These signaling molecules spread the inflammatory response.
Astrocytes, another type of glial cell, also release these inflammatory mediators. They contribute to neuroinflammation.
Inflammatory Mediators in the Brain
The release of cytokines and chemokines by microglia and astrocytes can have both good and bad effects on the CNS. These molecules can activate more immune cells. This leads to a chain of inflammatory events.
Understanding how these cells and mediators interact is key. It helps us see how neuroinflammation affects neurodegenerative diseases.
Blood-Brain Barrier and Inflammation
The blood-brain barrier is vital in controlling what enters the CNS. It keeps immune cells and inflammatory molecules out. If it’s disrupted, neuroinflammation worsens, leading to neurodegenerative disorders.
By studying neuroinflammation, we learn about its cellular components, mediators, and the blood-brain barrier. This knowledge helps us understand neurodegenerative diseases better. It also opens up new ways to treat them.
Neuroinflammation in Neurodegenerative Diseases
Neuroinflammation is a key player in many neurodegenerative diseases. It affects conditions like ALS, Huntington’s disease, Alzheimer’s disease, and Parkinson’s disease. Glial cells, especially microglia and astrocytes, get activated. This leads to inflammation that worsens the disease and causes neurons to die.
In ALS, the death of motor neurons is a major issue. This leads to a quick decline in health, often within 3 to 5 years. Current treatments like Riluzole and Edavarone only offer small benefits.
Huntington’s disease also sees a lot of inflammation. This affects motor skills, thinking, and behavior. It usually starts around age 45, with the early stages depending on genetic factors.
Alzheimer’s disease and Parkinson’s disease also have strong inflammatory responses. These responses contribute to the loss of neurons and decline in thinking. It’s important to understand how the immune system, metabolism, and genes work together in these diseases.
The role of inflammation in neurodegenerative diseases is clear. It shows why fighting inflammation could be a good treatment approach. More research is needed to find ways to slow or stop these diseases.
Molecular Mechanisms of Neuronal Damage
Neuroinflammation sets off a series of complex molecular processes that lead to severe neuronal damage in neurodegenerative diseases. A key process is the creation of reactive oxygen species (ROS) through oxidative stress. These molecules can directly harm neurons, causing neuronal loss.
Mitochondrial dysfunction is also a major factor in neurodegeneration. When neurons can’t produce energy properly and misfolded proteins build up, it makes cells more stressed and vulnerable. For example, in Huntington’s disease, the misfolded huntingtin (mHTT) protein causes inflammation, adding to the damage.
- Oxidative Stress Pathways: Neuroinflammation leads to the creation of harmful reactive oxygen species, causing direct damage to neurons and leading to neuronal loss.
- Mitochondrial Dysfunction: Problems with energy production and the buildup of misfolded proteins harm neurons and make them more likely to die.
- Protein Aggregation and Inflammation: The formation of protein aggregates, like mHTT in Huntington’s disease, triggers inflammation, which further damages neurons.
| Mechanism | Description | Impact on Neurons |
|---|---|---|
| Oxidative Stress | Neuroinflammation leads to the production of reactive oxygen species, causing direct cellular damage. | Leads to neuronal loss and impaired neuronal function. |
| Mitochondrial Dysfunction | Impaired energy production and the accumulation of misfolded proteins compromise neuronal health. | Contributes to neurodegeneration and neuronal loss. |
| Protein Aggregation | The formation of protein aggregates, such as mHTT in Huntington’s disease, can induce inflammatory responses. | Exacerbates neuronal damage and further drives the neuronal loss observed in neurodegenerative diseases. |
Understanding these complex molecular mechanisms helps researchers and clinicians create targeted treatments. These treatments aim to reduce the harm caused by neuroinflammation and help neurons survive in neurodegenerative disorders.
 How does chronic stress affect the functioning of the hippocampus?
How does chronic stress affect the functioning of the hippocampus?
Microglial Activation and Disease Progression
Microglial activation is key in the brain’s fight against neurodegenerative diseases. These brain cells can protect or harm, depending on how they’re activated. The balance between different types of microglial cells is important.
When activated, microglia release harmful cytokines and eat away at neurons. This neuroinflammatory response can cause more brain damage. It plays a big role in how fast and severe neurodegenerative diseases get.
In ALS, microglia eating dying motor neurons makes the disease worse. In Huntington’s disease, the brain’s immune cells get too active. This leads to more cytokine production and brain cell problems.
It’s vital to understand how microglial activation affects neurodegenerative diseases. By controlling these cells, we might slow down disease progression. Researchers are working on treatments that help these cells protect the brain.
Research is uncovering the complex role of microglia in neurodegenerative diseases. This knowledge could lead to new treatments. These treatments aim to use microglia’s protective abilities to fight disease.
Astrocytic Responses in Neurodegeneration
Astrocytes are the most common glial cells in the brain. They play a key role in neuroinflammation and neurodegeneration. Astrocytes become reactive and produce more GFAP in diseases like Alzheimer’s and Parkinson’s.
Reactive Astrogliosis
Reactive astrogliosis is how astrocytes respond to brain injuries. They change shape and function, forming a glial scar. This scar tries to protect the brain but can also block healing.
Neurotrophic Factor Production
Astrocytes also make important neurotrophic factors like BDNF and GDNF. These help keep neurons healthy. But, how much is too much, and how does it affect the brain?
Glial Scar Formation
The glial scar formed by reactive astrocytes has both good and bad sides. It can stop inflammation but also block nerve fiber growth. This makes recovery harder.
It’s important to understand astrocytes’ role in neurodegeneration. We need to find ways to use their activation for good. This could help protect the brain and reduce inflammation.
Cytokine Signaling in Neural Tissue
Cytokine signaling is key in the neuroinflammatory cascade of many neurodegenerative diseases. Pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6 are high in diseases such as ALS and Huntington’s. These cytokines start and keep inflammation going, harming neurons and their survival.
It’s vital to understand cytokine signaling in the brain for new treatments. Cytokines affect microglial activation, synaptic plasticity, and neural circuit development. When these signals go wrong, it can harm neurodevelopment and lead to diseases like multiple sclerosis.
The complement system plays a big role in neuroinflammation and neurodegeneration. It helps keep the brain balanced. But, when the central nervous system gets invaded by immune cells, it can cause a lot of damage.
Trying to target cytokine networks has shown promise in early studies. But, moving these findings to real-world treatments has been tricky. This shows how complex cytokine signaling is and how much more research is needed.
| Cytokine | Role in Neuroinflammation | Associated Neurodegenerative Diseases |
|---|---|---|
| TNF-α | Initiates and perpetuates inflammatory cascades | ALS, Huntington’s disease |
| IL-1β | Contributes to neuronal damage and dysfunction | ALS, Huntington’s disease |
| IL-6 | Involved in the neuroinflammatory response | ALS, Huntington’s disease |
In summary, cytokine signaling is crucial for understanding neuroinflammation in neurodegenerative diseases. Research is ongoing to understand how cytokines, the complement system, and the blood-brain barrier work together. The goal is to find better treatments for the neuroinflammatory cascade.
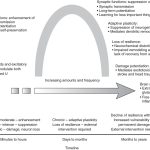
Impact of Chronic Inflammation on Brain Health
Chronic inflammation in the brain can harm your brain health and function. It can change how your brain learns and remembers things. This can lead to cognitive decline seen in diseases like Alzheimer’s and Parkinson’s.
Synaptic Plasticity Changes
Inflammation in the brain can mess with synapses, the connections between neurons. This can alter how synapses work, affecting learning and memory. Such changes can make it harder to think and remember things.
Neuronal Circuit Disruption
Chronic inflammation can also mess with the brain’s complex circuits. These circuits are key to many brain functions. Research is ongoing to understand how long-term inflammation affects the brain.
Understanding how chronic inflammation affects the brain is key. It helps in finding ways to keep our brains healthy and prevent neurodegenerative diseases. By tackling inflammation, we can improve brain health and quality of life.
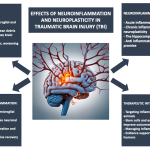 Neuroplasticity and Cognitive Recovery After Traumatic Brain Injury
Neuroplasticity and Cognitive Recovery After Traumatic Brain Injury
Biomarkers of Neuroinflammation
Finding good biomarkers for neuroinflammation is key for early disease detection and tracking. Neurofilament light chain (NfL) is seen as a top biomarker for diseases like Huntington’s. Imaging tools, like PET scans, show brain inflammation. These tools help in understanding disease stages and treatment effects.
A study with 125 people from Alzheimer’s prevention studies found something interesting. Those with Alzheimer’s had higher levels of calprotectin, a sign of inflammation. Other Alzheimer’s biomarkers also rose with inflammation levels. Memory scores fell with higher calprotectin, affecting both diagnosed and undiagnosed Alzheimer’s patients.
This research points to gut inflammation causing brain inflammation and damage. Scientists are testing mice to see if diet changes can cause Alzheimer’s-like symptoms. They want to know if diet changes linked to inflammation can lead to Alzheimer’s symptoms in mice.
| Biomarker | Application | Relevance |
|---|---|---|
| Neurofilament light chain (NfL) | Monitoring neurodegenerative diseases like Huntington’s | Emerging as a promising biomarker for early diagnosis and treatment evaluation |
| Microglial activation (PET scans) | Assessing inflammatory status of the brain | Provides insights into the neuroinflammatory process |
| Calprotectin (fecal test) | Detecting gut inflammation in Alzheimer’s disease | Linked to increased Alzheimer’s biomarkers and cognitive decline |
These biomarkers are vital for early detection, tracking, and managing neurodegenerative diseases. They open the door for more focused and tailored treatments.
Therapeutic Approaches Targeting Inflammation
Targeting neuroinflammation is a promising way to fight neurodegenerative diseases. Anti-inflammatory drugs, like PARP1 inhibitors, have shown great promise in early studies. They can change how inflammation works and protect the brain.
Anti-inflammatory Medications
PARP1 inhibitors are known for their anti-inflammatory effects. They have shown great promise in early studies on neurodegenerative diseases. These drugs can stop inflammation, reduce damage, and protect the brain.
Novel Treatment Strategies
Researchers are also looking into new ways to treat diseases. They want to use the body’s own ways to fight inflammation and protect the brain. This includes using immunomodulation and neuroprotection.
Using a mix of treatments that target different parts of the disease could be very effective. This approach could help with diseases like Huntington’s and ALS. It aims to tackle the disease from many angles at once, which could lead to better results for patients.
| Therapeutic Approach | Mechanism of Action | Potential Applications |
|---|---|---|
| PARP1 Inhibitors | Suppress inflammatory pathways, reduce oxidative stress, promote neuroprotection | Neurodegenerative diseases, including Huntington’s disease and ALS |
| Immunomodulation Strategies | Harness the body’s regulatory mechanisms to mitigate neuroinflammation | Neurodegenerative diseases, autoimmune disorders |
| Combination Therapies | Target both genetic factors and inflammatory processes for synergistic benefits | Huntington’s disease, ALS, and other complex neurodegenerative conditions |
By exploring new ways to fight neuroinflammation, researchers hope to find new ways to manage and maybe even prevent neurodegenerative diseases. This could be a big step forward in treating these serious conditions.
Genetic Factors Influencing Neuroinflammation
Genetic variations are key in how our brains react to inflammation in diseases like Parkinson’s and Alzheimer’s. Some genes control our immune system and how we fight inflammation. Knowing these genes helps doctors tailor treatments and spot who might get sicker faster.
In Huntington’s disease, the number of CAG repeats in the HTT gene affects when and how bad the symptoms get. People with 36 to 39 repeats might get the disease, but those with 40 or more will definitely show symptoms. The CAP score, which combines these repeats and age, usually shows symptoms around 45 and is about 100.
Alzheimer’s disease also has a genetic link, with the APOE gene being a big risk factor. Research shows that too many CAG repeats in certain cells can cause motor symptoms in HD.
Genetic factors also shape the inflammatory response in these diseases. Studies show that inflammation is a big part of HD, with immune cells like microglia and astrocytes playing key roles.
Grasping the genetic causes of neuroinflammation is vital for better treatments and early detection. By focusing on these genetic factors, scientists and doctors can find new ways to help patients and prevent disease progression.
Prevention Strategies and Lifestyle Factors
Managing neuroinflammation can help prevent neurodegenerative diseases. By making lifestyle changes, you can reduce brain inflammation. This approach can protect your brain health.
Diet and Inflammation
An anti-inflammatory diet is key. It should include omega-3 fatty acids, antioxidants, and other brain-protective foods. Eating fatty fish, berries, leafy greens, and nuts can help reduce inflammation and boost brain function.
Exercise and Neuroinflammation
Regular physical activity is beneficial for neuroinflammation. Exercise lowers inflammatory mediators and boosts anti-inflammatory cytokines. It also helps the brain recover from inflammation.
Combining an anti-inflammatory diet with regular physical activity supports neuroprotection. This can help maintain brain health. These lifestyle changes can work alongside medical treatments to manage neurodegenerative disorders and improve cognitive function.
Future Directions in Research and Treatment
Our understanding of neuroinflammation and its role in neurodegenerative diseases is growing. This growth brings hope for the future of research and treatment. Personalized medicine is becoming a key focus. It uses early intervention based on inflammatory biomarkers to offer treatments that fit each person’s needs.
 What are the most innovative topics in neuroscience today?
What are the most innovative topics in neuroscience today?
Combination therapies are also showing promise. They target different parts of neuroinflammation at the same time. This approach aims to provide better treatment results and possibly slow down or stop neurodegenerative diseases from getting worse.
Understanding the link between genetics, environment, and inflammation is vital. This knowledge will help create more effective treatments and prevention strategies. As research continues, we can look forward to better lives for those with these conditions.






