Organometallic Chemistry: Metal-Carbon Bonding and Its Applications

In the field of organometallic chemistry, metals and carbon compounds are key. This area studies the special bonds between them. It changes how we see catalysis, organic synthesis, and materials science.
It helps solve big problems like indoor air pollution. It also opens up new ways to make advanced materials. Organometallic chemistry is key to many new discoveries today.
At the core of organometallic chemistry is the study of Organometallic Chemistry Applications. It’s about how metals and carbon molecules work together. This leads to new solutions.
One example is using Metal-Carbon Bonds to make materials that clean the air. These materials, like metal-organic frameworks (MOFs), are very good at removing pollutants. They could greatly improve our air quality indoors.
Understanding Metal-Carbon Bonds: Fundamentals and Properties
Metal-carbon bonds are key in organometallic chemistry. They are the base for many uses. These bonds come in different types, like sigma and pi bonds, each with its own electronic structure and how it reacts.
Knowing about metal-carbon bonds is vital. It helps in making better catalysts, materials, and solutions for everyday problems.
Types of Metal-Carbon Bonds
Metal-carbon bonds can be divided into several types. Here are a few:
- Sigma (σ) Bonds: These bonds are common. They form when a metal’s s or d orbital mixes with a carbon’s sp3 orbital.
- Pi (π) Bonds: These bonds happen when a metal’s d orbitals mix with a carbon’s p orbitals. This creates a more complex bond.
- Allyl and Benzyl Bonds: These bonds form when a metal connects to the π-system of unsaturated hydrocarbons. This makes stable organometallic complexes.
Electronic Structure and Bonding Mechanisms
The electronic structure of metal-carbon bonds depends on several things. These include the metal’s oxidation state, the carbon ligand, and other ligands around it. These factors affect the bond’s strength, polarity, and how reactive it is.
Understanding how these bonds form is key. It helps predict and change the properties of these compounds.
Stability and Reactivity Patterns
The stability and reactivity of metal-carbon bonds depend on several factors. These include the metal’s electronic setup, the carbon ligand, and other ligands. These factors affect how stable the bond is and how it reacts to different reactions.
Designing effective catalysts and materials requires careful thought. It’s about balancing electronic, steric, and kinetic factors.
Understanding metal-carbon bonds is essential. It opens up new possibilities in organometallic chemistry. This includes solving problems like formaldehyde capture, catalysis, and making new medicines. By mastering these concepts, we can innovate and create sustainable solutions for a better future.
Historical Evolution of Organometallic Chemistry
Organometallic chemistry has changed a lot since it began. It started with simple compounds like CCl4 and CFCl3. Now, it explores how metals and carbon-based molecules interact. This has helped solve big environmental problems, like breaking down PFCs and making air cleaner.
The chemical developments in organometallic chemistry history have led to big steps in catalysis and materials science. Scientists have learned a lot about metal-carbon bonds. This knowledge has made organometallic compounds more stable and useful, changing many industries and medicines.
The journey from simple metal-carbon bonds to green solutions shows the power of science. As organometallic chemistry keeps growing, we’ll see even more amazing breakthroughs. These will help shape our future.
Key Synthetic Methods in Organometallic Chemistry
Synthetic methods in organometallic chemistry are key to making new materials. These include metal-organic frameworks (MOFs) for environmental solutions. The main methods are direct metal-carbon bond formation, transmetallation reactions, and oxidative addition pathways.
Direct Metal-Carbon Bond Formation
Direct metal-carbon bond formation is a basic technique. It uses organometallic reagents like organolithium or Grignard compounds. These react with metal halides or other metal precursors.
The result is a metal-carbon bond. This bond can then be modified or used in complex molecules.
Transmetallation Reactions
Transmetallation reactions move an organic group from one metal to another. This creates new metal-carbon bonds. It’s great for adding specific groups to metal centers.
 Bioinorganic Chemistry: Metals in Biological Systems
Bioinorganic Chemistry: Metals in Biological Systems
This makes a wide range of organometallic compounds possible.
Oxidative Addition Pathways
Oxidative addition is a key step in organometallic chemistry. It involves a metal inserting into a bond, like carbon-halogen or carbon-hydrogen. This increases the metal’s oxidation state and coordination number.
It leads to new metal-carbon bonds. This opens up many synthetic opportunities.
These methods are vital for making advanced materials. For example, Al-3.5-PDA (MOF-303) shows their importance. It’s used for capturing formaldehyde and other pollutants.
Using these methods together has advanced organometallic chemistry. It has led to more complex and functional materials. These have many applications.
Organometallic Chemistry Applications in Modern Industry
Organometallic chemistry is key in driving innovation across many industries. It’s used in catalysis and organic synthesis, meeting the needs of today’s industry.
In catalysis, organometallic compounds are very useful. They help make chemical processes more efficient and green. For example, they help capture and remove formaldehyde, a harmful indoor pollutant, using metal-organic frameworks (MOFs).
Organometallic catalysts also help in making advanced materials for environmental remediation. Scientists have created catalysts to break down perfluorinated compounds (PFCs), harmful pollutants. These catalysts use metal-carbon bonds to help clean our environment.
In organic synthesis, organometallic chemistry opens up new ways to make complex molecules. This is very helpful in the pharmaceutical industry. It’s used to make many medicines and other important compounds.
The wide range of uses for organometallic chemistry shows its huge potential. As research keeps going, we’ll see even more important uses. These will help solve big industrial and environmental problems, leading to a better future.
Catalysis and Reaction Mechanisms
Organometallic chemistry is key in catalysis, covering both homogeneous and heterogeneous systems. Catalysts made from organometallic compounds are vital for many industrial and environmental processes. They show great efficiency in different reactions, often better than traditional methods.
Homogeneous Catalysis
Homogeneous catalysis focuses on the same phase for catalysts and reactants. Organometallic catalysts help control complex reactions, improving product quality. For instance, they efficiently break down perfluorocarbons (PFCs) like CF4 at lower temperatures than traditional methods.
Heterogeneous Catalysis Systems
Organometallic chemistry is also vital for heterogeneous catalysis, where phases differ. These catalysts are designed for specific reactions, offering stability and recyclability. They are used in many industries, from refining to environmental cleanup.
Organometallic catalysts are crucial in today’s industries and environmental efforts. As organometallic chemistry grows, so will the focus on better, greener catalytic systems. This will lead to new innovations across many fields.
Role in Pharmaceutical Development
In the world of making new medicines, organometallic chemistry is key. It helps create new drug compounds. Metal-carbon bonds allow for complex structures, vital for new medicines.
This field helps make new medicines and improve how drugs are made. It’s a big help in the pharmaceutical world.
Organometallic chemistry is used in many ways in medicine. It’s used in Pharmaceutical Applications and in Drug Synthesis. It lets researchers make drugs that are more precise and effective.
This leads to better treatments and better health for patients. It’s a big step forward in medicine.
| Application | Description |
|---|---|
| Pharmaceutical Applications | Organometallic compounds are used in various stages of drug development, including the synthesis of active pharmaceutical ingredients (APIs), the design of drug delivery systems, and the development of diagnostic imaging agents. |
| Drug Synthesis | The unique reactivity of metal-carbon bonds allows for the efficient construction of complex molecular structures, enabling the synthesis of novel drug candidates and the optimization of existing drug manufacturing processes. |
Organometallic chemistry opens new doors in drug making. It leads to better care and meets medical needs. The future of medicine looks bright thanks to this field.
Environmental Applications and Green Chemistry
Organometallic chemistry is key in solving environmental problems and promoting green chemistry. It helps create eco-friendly ways to make things and find new ways to clean pollution. This makes it a crucial part in tackling big environmental issues.
 Heterogeneous Catalysis in Inorganic Synthesis
Heterogeneous Catalysis in Inorganic Synthesis
Sustainable Synthesis Methods
Organometallic chemistry helps make sustainable synthesis possible. For example, metal-organic frameworks (MOFs) can grab and hold bad stuff like formaldehyde. This is a big problem indoors. MOFs use special bonds to catch and get rid of pollutants, making factories cleaner.
Pollution Control Solutions
Organometallic compounds are also used to solve pollution problems. Catalysts made from these compounds can break down harmful gases like PFCs. These gases are bad for the environment and contribute to climate change. The special bonds in these catalysts help them work well, reducing pollution.
Organometallic chemistry shows it can help solve big environmental issues. Green Chemistry and Sustainable Synthesis methods, along with Pollution Control solutions, are changing the future for the better. They make our world more environmentally friendly.
Advanced Materials and Nanotechnology
Organometallic chemistry is key in making advanced materials and nanotechnology. Metal-organic frameworks (MOFs) are a great example. They show the amazing properties and uses of these new materials. MOFs, like Al-3.5-PDA (MOF-303), mix organometallic chemistry with the latest in materials science and nanotechnology.
Biopolymers, like polylactic acid (PLA), are getting a lot of attention for medical uses. They are good for things like tissue engineering and drug delivery. Their big surface area makes them great for sensors and filters too.
Solution blow spinning (SBS) is a new way to make nanofibers. It’s been getting more research since 2014. SBS is fast and can make nanofibers without a strong electric field, which is useful for many uses.
Silver nanostructures, made by chemical reduction, are very good at fighting germs. They are used in water and air cleaning, medicine, and healing wounds. Adding silver nanoparticles to wound dressings helps them work better, especially for diabetic ulcers and burns.
| Material | Key Characteristics | Applications |
|---|---|---|
| Polylactic Acid (PLA) |
|
|
| Ag2CO3 Nanoparticles |
|
|
Advances in organometallic chemistry, materials science, and nanotechnology are leading to new solutions. These are in fields like medicine and green energy. As research keeps growing, we’ll see even more amazing discoveries soon.
Analytical Methods and Characterization Techniques
Organometallic chemistry uses advanced methods to study metal-carbon bonds and unique compounds. Key areas include spectroscopic analysis and structure determination.
Spectroscopic Analysis
Techniques like NMR, IR spectroscopy, and mass spectrometry give deep insights. They help identify molecular structures and bonding patterns. These tools also show the metal’s oxidation state and the metal’s coordination environment.
Comprehensive two-dimensional gas chromatography (GC×GC) with TOF-MS is now widely used. It offers better separation and identification. This helps researchers quickly get detailed data on their samples.
Structure Determination Methods
X-ray crystallography is key in organometallic chemistry. It shows the exact arrangement of atoms in compounds. This gives a clear picture of how structure affects properties and reactivity.
New materials and catalysts development depends on these insights. Researchers use software and workflow optimization. This helps them understand the structure and behavior of these complex systems.
Recent Developments and Future Prospects
Organometallic chemistry has seen big leaps forward in recent years. Researchers are pushing the boundaries and finding new uses. They’re making better and more precise catalysts for many industrial processes. Emerging technologies, like advanced materials and nanotechnology, are bringing new chances for organometallic compounds.
The future of organometallic chemistry looks bright and varied. Scientists are digging deeper into how metals and carbon interact. They aim to understand these interactions better to open up new discoveries. New research trends are looking into fresh ways to make organometallic compounds and their uses.
One exciting area is using organometallic compounds for better energy storage and conversion. Scientists are working on new battery technologies, fuel cells, and ways to make renewable energy. They’re also focusing on using organometallic chemistry to clean up pollution and make sustainable products.
As organometallic chemistry grows, we’ll see the creation of groundbreaking materials, catalysts, and technologies. These will change many industries. By combining organometallic science with materials science and nanotechnology, we’ll find new ways to solve today’s big challenges.
Safety Considerations and Laboratory Practices
When working with Laboratory Safety and Handling Organometallic Compounds, safety is key. These materials can be very reactive. So, it’s important to handle, store, and dispose of them properly.
This keeps everyone safe and makes sure the lab is a safe place to work. Following strict safety rules is vital to avoid accidents and keep the team healthy.
Make sure to wear the right personal protective equipment (PPE). This includes lab coats, safety glasses, and gloves that can resist chemicals. Also, your lab should have good ventilation to deal with risks from air-sensitive and pyrophoric compounds.
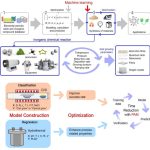 Advanced Inorganic Materials: Synthesis and Properties
Advanced Inorganic Materials: Synthesis and Properties
Plan your experiments carefully to reduce risks. This way, you can work safely with these materials. It’s all about being careful and prepared.
By focusing on safety and good lab practices, you can explore organometallic chemistry safely. You’ll be able to make new discoveries while keeping risks low. Always be careful, stay informed, and keep yourself safe in this exciting field.





